Executive Summary
Safety Intelligence reports provide insights into safety of biologics and therapeutics drug products.
Adverse Event Reporting intelligence contains Medical Dictionary for Regulatory Activities - MedDRA Codes, Common Terminology Criteria for Adverse Events (CTCAE) and National Drug Code (NDC) are associated with every adverse event. It adheres to the international safety reporting guidance issued by the International Conference on Harmonisation (ICH E2B). Using the intelligence database you can identify the cases, problems, suspect drugs or suspect devices, route of administration adopting ICH List & codes, delivery dosages, therapy performed, manufacturer information and other useful data.
Adverse Event Reporting System data includes the reports submitted to FDA by Foreign, Health professional, Consumer, Distributor, Literature, Company representative, User facility, Study and Others. Adverse Event Reporting Intelligence reports are based on ten years of AERS data.
Features
Key features of Adverse Event Reporting Intelligence include:
-
Provide Adverse Event reports
-
Provision individual case safety reports
-
Access Information on adverse event & medication error reports submitted to FDA
-
Patient Demographics and Administrative information
-
Drug information and Reaction information
-
Medication Error Reports
-
Medical Dictionary for Regulatory Activities (MedDRA) codes and National Drug Code (NDC) information, ICH List & Codes
-
Deep insights into safety concerns of the marketed product
-
Detect & Interpret potential signals of Serious Risks
-
Enforcement Reports - Product Recalls (Drug recalls) and Market Withdrawals
-
Drug Class
-
Reaction Information
-
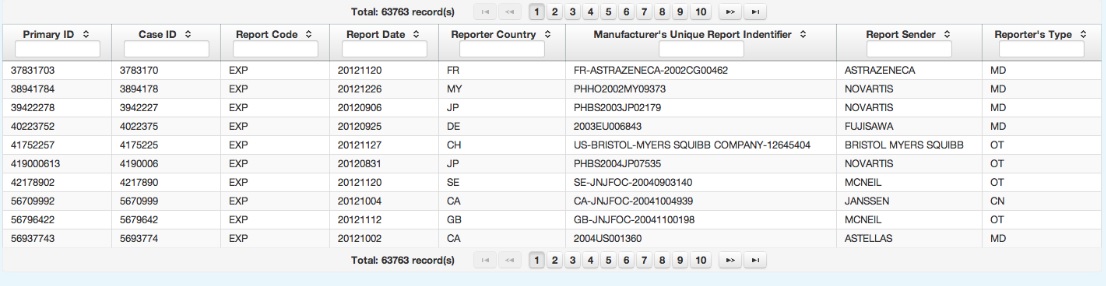
Adverse Events Case Reports
-
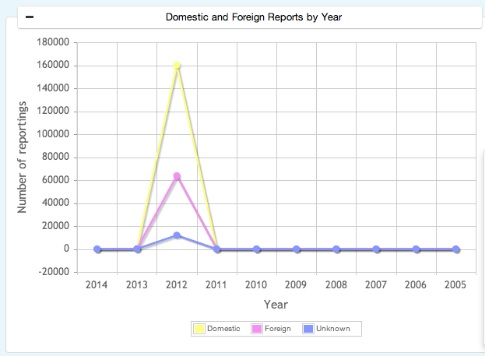
Domestic & Foreign Cases information reports
-
Administrative information Reports
-
Patient outcomes & Patient Demogrpahics
-
Primary, Secondary and other suspects
-
Drug Therapy information
-
Enforcement Reports - Product Recalls and Market Withdrawals
-
Enforcement Reports based on Product Types - Drugs, Biologics, Devices, Food, Cosmetics and Veterinary
-
MedDRA codes and National Drug Code (NDC) information, ICH List & Codes, Product Definitions, Package Definitions
Adverse Event Reporting
Some of the Safety Intelligence - Adverse Event Reporting analytics reports include:


