Drug Product Intelligence
VajraSoft Inc. Pintels - Patent Intelligence Systems - BioPharMed IP Intelligence is most comprehensive competitive IP Intelligence provisioning system. Pintels delivers Intelligence Analytics on Demand. A unique aspect of Pintels is that it offers end-to-end intelligence analytics - starting from IP / Patent Intelligence, to products to it's Market intelligence including the post-market performance, safety intelligence, Adverse Event reports, Medication error reports, Patient outcomes and Drug Master Files information.
Business Challenges - Business Executives are constantly challenged to gain competitive product intelligence to get right insights and make right decisions at right time to provide more "value" and improve stakeholder value. That said and done - how to achieve it? One of the key aspect is to gain deep insights into Patent landscape, products (biologics and therapeutics drugs), drug classes, active ingredients, dosage and delivery methods. In addition provide manufacturer information and Drug Master File (DMF) information. One of the unique value proposition of Pintels is that it not only provides product details, but show cases the related patent information as well as post market product safety that includes any adverse events reporting intelligence, recalls & withdrawals, Enforcements reports providing end-to-end drug product intelligence and improve stakeholder value.
Product Intelligence Solutions
These intelligence solutions include:
-
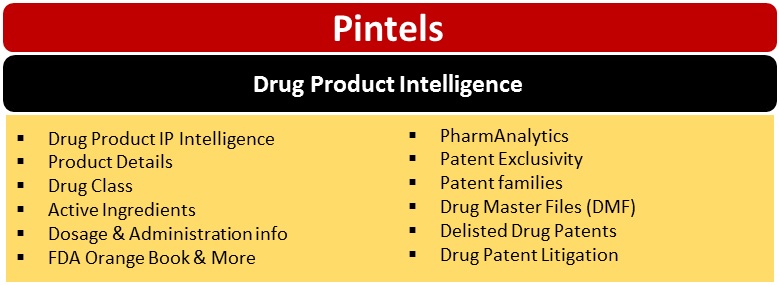
Drug Product Intelligence
-
Drug Class
-
Active Ingredients
-
PharmAnalytics
-
Drug Master Files
-
Paragraph IV Patent Certifications
-
Drug Patent Litigation
-
FDA-approved brand name and generic prescription drugs
-
Over-the-counter (OTC) human drugs
-
Discontinued drugs (DISC)
-
Labels for approved drug products
-
Generic drug products
-
Drugs with a specific active ingredient
-
View the drug approval history
-
Tentative Approvals
-
Chemical Type 6 approvals
-
Links to drug safety and patient information
You can search:
You can search by - drug name, active ingredient, drug name and FDA Action Date range, application number (NDA, ANDA, BLA), action dates of approvals.
The search results for all drug products include - drug name, active ingredient, dosage form or route of administration, strength, marketing status (prescription, over-the-counter, or discontinued), company that sponsored an application for approval, FDA action date, approval type.
You can also find:


